
At Praxis, we are committed to changing the future for those diagnosed with Developmental and Epileptic Encephalopathy (DEE).
At Praxis, we are committed to changing the future for those diagnosed with Developmental and Epileptic Encephalopathy (DEE).
DEE refers to a group of epilepsies that are characterized by both seizures as well as encephalopathy, which is a term used to describe developmental delay or loss of developmental skills.
We are excited to be investigating 2 first-in-class potential DEE treatments, Relutrigine (PRAX-562) and Elsunersen (PRAX-222), both of which are purposefully developed to reduce seizures and improve quality of life for those living with a DEE.
Our Resilience program is comprised of 3 unique clinical trials, each intended to study the impact of our potential treatments in people diagnosed with DEEs. Click below to learn more about each trial.

EMBOLD Study
(Relutrigine)
for children diagnosed with early-onset SCN2A or SCN8A DEE

EMERALD Study
(Relutrigine)
for children and adults diagnosed with any DEE and related syndrome
Learn more about our 2 potential treatments for children and adults with DEEs

Relutrigine
Relutrigine is an investigational medicine that can be taken orally or administered through a G/J tube. Relutrigine is designed to regulate sodium flow in brain cells by targeting overactive sodium channels that cause seizures; therefore, potentially offering better seizure control with fewer side effects. Relutrigine has been designed to maximize its effects against overactive sodium channels that are believed to cause seizure activity while minimizing the blocking of normal activity needed for healthy brain function.
Elsunersen
Elsunersen is an investigational antisense oligonucleotide (ASO) administered monthly by intrathecal injection. Elsunersen has the potential to be the first disease-modifying treatment for early-onset gain-of-function SCN2A DEE, designed to selectively decrease SCN2A gene expression. By targeting the underlying genetic cause of disease, elsunersen has demonstrated potential to go beyond seizures to treat other symptoms of the disease.
Study Guide Quick Comparison
| Criteria | EMBOLD Study Recruitment Complete | EMBRAVE 3 Study | EMERALD Study |
|---|---|---|---|
| SCN2A DEE | | | |
| SCN8A DEE | | | |
| All DEE diagnoses | | | |
| Age | 1-18 years old | 0-18 years old | 2-65 years old |
| Minimum of 4 seizures per month | | | |
| Minimum of 8 seizures per month | | | |
Location Options
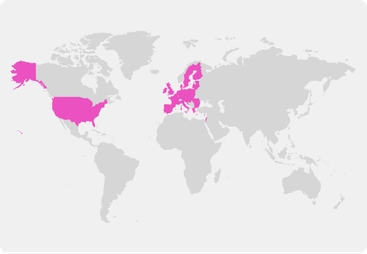
Choice of at-home, in-clinic, or hybrid study participation
Locations:
Recruitment Complete
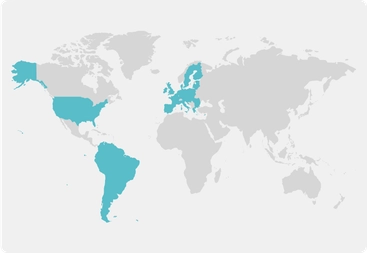
In-clinic participation
Locations:
Europe
South America
United Kingdom
United States
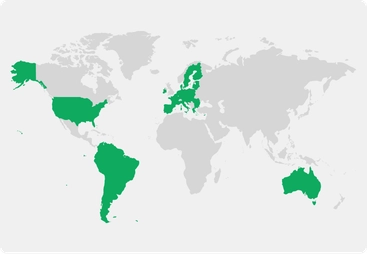
Choice of at-home, in-clinic, or hybrid study participation
Locations:
Australia
Europe
South America
United States
