

Revolutionizing the standard of care for children with early-onset SCN2A Developmental and Epileptic Encephalopathy (DEE)
The EMBRAVE 3 study is currently enrolling to evaluate a new potential treatment targeting the underlying cause of disease in early-onset SCN2A DEE.
About the EMBRAVE 3 Study

Purpose
To understand how safe and effective elsunersen is in reducing seizures and improving other symptoms associated with early-onset SCN2A DEE

Duration
Up to 24 weeks in the initial study, with the opportunity to continue treatment for an additional 24 weeks

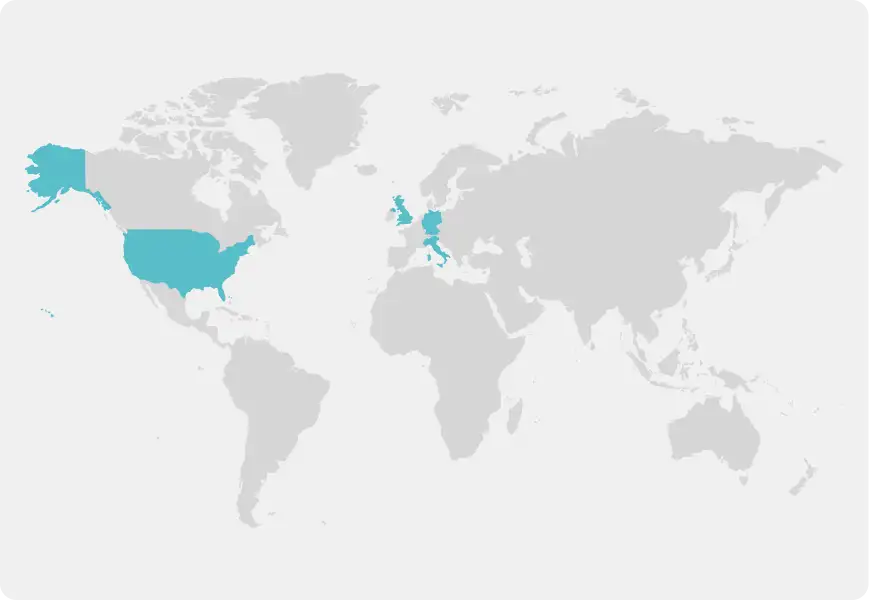
In Clinic
United States, Italy, Germany and United Kingdom
About Elsunersen
Elsunersen is an investigational antisense oligonucleotide (ASO) with the potential to be the first disease-modifying treatment for early-onset SCN2A DEE, designed to selectively decrease SCN2A gene expression. By targeting the underlying genetic cause of disease, elsunersen has demonstrated potential to go beyond seizures to treat other symptoms of the disease.
About ASOs
ASOs are short, lab-made strands of genetic material that can target specific genes and adjust how they behave. They are custom-designed to match the genetic error causing the disease. That means they don’t just treat symptoms, they go after the root cause. Elsunersen, a type of ASO, is designed to reduce the levels of SCN2A gene expression. By lowering how much of the SCN2A protein is made, elsunersen helps quiet the excessive electrical activity in the brain, which may reduce the seizure burden and improve quality of life for children diagnosed with early-onset SCN2A DEE.

EMBRAVE 3 Study Key Eligibility Criteria
-
 Are 0 through 18 years old
Are 0 through 18 years old -
 Have received a diagnosis of a SCN2A gene mutation with onset of seizures in the first 3 months of life
Have received a diagnosis of a SCN2A gene mutation with onset of seizures in the first 3 months of life -
 Have at least 4 motor seizures (seizures that involve movement) in the 4 weeks prior to screening
Have at least 4 motor seizures (seizures that involve movement) in the 4 weeks prior to screening
Why should my child participate?
-
 Every participant in this study will be assigned to receive elsunersen—there is no placebo group
Every participant in this study will be assigned to receive elsunersen—there is no placebo group -
 The study is designed to reduce the burden of participation by offering a combination of in-clinic visits and at-home telehealth visits
The study is designed to reduce the burden of participation by offering a combination of in-clinic visits and at-home telehealth visits -
 Travel assistance is available. This will include lodging, meals, and any other costs associated with study participation will be paid for by the sponsor
Travel assistance is available. This will include lodging, meals, and any other costs associated with study participation will be paid for by the sponsor -
 Opportunity to potentially change the future for children diagnosed with SCN2A DEE
Opportunity to potentially change the future for children diagnosed with SCN2A DEE
